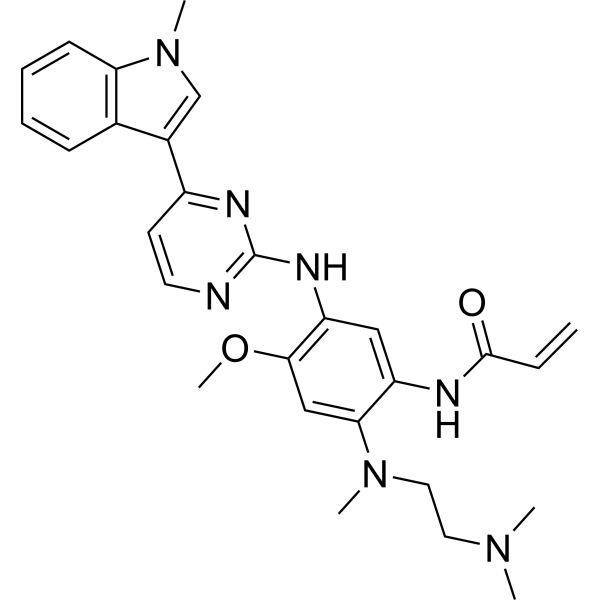
| 英文名稱 | AZD-9291 (Osimertinib; Mereletinib) |
|---|---|
| 中文名稱 | 奧希替尼/邁瑞替尼 |
| CAS號(hào) | 1421373-65-0 |
| 分子式 | C28H33N7O2 |
| 分子量 | 499.62 |
| 外觀 | Off-white powder |
| 儲(chǔ)存條件 | Dry, dark and at 0 - 4 ℃ for short term (days to weeks) or -20 ℃ for long term (months to years). |
- 021-58180488
- sales@MedChemLeader.com
-
抑制劑信號(hào)通路研究Autophagy | 自噬 Angiogenesis Apoptosis | 細(xì)胞凋亡 Cell Cycle | 細(xì)胞周期 Cytoskeletal Signaling | 細(xì)胞骨架 DNA Damage/DNA Repair Endocrinology & Hormones Epigenetics | 表觀遺傳學(xué) GPCR & G Protein Immunology & Inflammation MAPK/ERK Pathway Membrane Transporter/Ion Channel Metabolism Microbiology/Virology/ Anti-infection Neuronal Signaling | 神經(jīng)信號(hào)通路 NF-κB PI3K/Akt/mTOR Proteases Protein Tyrosine Kinase/RTK Stem Cells & Wnt | 干細(xì)胞及 Wnt 通路 TGF-beta/Smad | 信號(hào)通路 Vitamin D Related Antibody-drug Conjugate/ADC Related PROTAC JAK/STAT 信號(hào)通路 Ox Stress Reagents(OX應(yīng)激試劑)
-
凱立德生物商城首頁(yè)
-
10000+產(chǎn)品中心
- 信號(hào)通路>
- Autophagy | 自噬
- Angiogenesis
- Apoptosis | 細(xì)胞凋亡
- Cell Cycle | 細(xì)胞周期
- Cytoskeletal Signaling | 細(xì)胞骨架
- DNA Damage/DNA Repair
- Endocrinology & Hormones
- Epigenetics | 表觀遺傳學(xué)
- GPCR & G Protein
- Immunology & Inflammation
- MAPK/ERK Pathway
- Membrane Transporter/Ion Channel
- Metabolism
- Microbiology/Virology/ Anti-infection
- Neuronal Signaling | 神經(jīng)信號(hào)通路
- NF-κB
- PI3K/Akt/mTOR
- Proteases
- Protein Tyrosine Kinase/RTK
- Stem Cells & Wnt | 干細(xì)胞及 Wnt 通路
- TGF-beta/Smad | 信號(hào)通路
- Vitamin D Related
- Antibody-drug Conjugate/ADC Related
- PROTAC
- JAK/STAT 信號(hào)通路
- Ox Stress Reagents(OX應(yīng)激試劑)
- 生命科學(xué)>
- 分子生物學(xué)
- 醫(yī)藥開(kāi)發(fā)&藥物發(fā)現(xiàn)研究
- 細(xì)胞生物學(xué)
- 生物化學(xué)及試劑
- 糖科學(xué)|糖類化合物
- 雜質(zhì)與代謝物
- 氨基酸及其衍生物
- 多肽與蛋白
- Enzyme(酶)
-
精美商品積分商城
-
技術(shù)服務(wù)
-
關(guān)于我們
-
聯(lián)系我們
-
新聞中心